What is Electric Charge?
An electric charge is the physical property of subatomic particles.
We can broadly classify the properties of matter into two different types. The first is a physical property, and the second is a chemical property. An electric charge is the physical property of particles which are subatomic in nature.
Now, what do we mean by subatomic, as the name is suggesting the anti-particles which are smaller than the size of an atom, and they exist within the atom, we are mainly talking about the two particles which are electrons And Protons.
we will mostly deal with the circuits in which only electron flow is relevant.
So this means we will focus on the flow of negative charge, in many devices and circuits the flow of positive charge is also relevant.
Like in the case of semiconductor devices, the flow of positive charge is also relevant along with the negative charge flow. But there the positive charge is due to the presence of holes. But in the case of network theory, we don’t have to worry about holes, because holes are not present inside the metals, and are present inside the semiconductors. And therefore, in network theory, the positive charge will be due to protons
Charge Is the Quantized
quantization of charge mean charge cannot exist in an arbitrary manner. This means you cannot have 1/2 e as the charge or 3/2 e as the charge. The charge cannot exist like this, because the charge is quantized.
the charge will exist as an integral multiple of e, where “east “e” is known as the elementary charge, and east is equal to 1.6 * 10-19 Coulomb. So we can see that the elementary charge is like the smallest packet of the charge, and we cannot divide this packet further.
Therefore, 1/2 e is not possible because this is equal to 0.5 e. And in this case, we are dividing the packet into 2 equal parts, which is not possible. Similarly, 3/2 e is also not possible, because this is equal to 1.5 E. And we can write 1.5 E equal to 1 E and 0.5 E.
we can have only an integral multiple of “e”. This means the charge will always be equal to N times “e”, where “N” is an integer, and the charge one is equal to 1.6 * 10-19 Coulomb.
what is Coulomb?
Coulomb is the SI unit of the charge, and it is named after French physicist Charles Coulomb. The charge can be of two types, the positive charge and the negative charge. Electrons have a negative charge, and protons have a positive charge,
charge of 1 electron is equal to the elementary charge with a negative sign because I told you electrons have a negative charge. This means electrons will have charge
e=-1.6*10-19 C
charge on one proton is equal to e. This makes the charge equal to 1.6*10-19C.
Now we will move on to the last point, which is an important point. This point is not at all important for your exam. This is important if you want to have good knowledge according to this point.
Coulomb is a very large unit.
This point is very straightforward forward Coulomb is a very large unit. But now we will understand this point, or you can say, now we will feel this point, why Colomb is a very large unit with the help of examples.
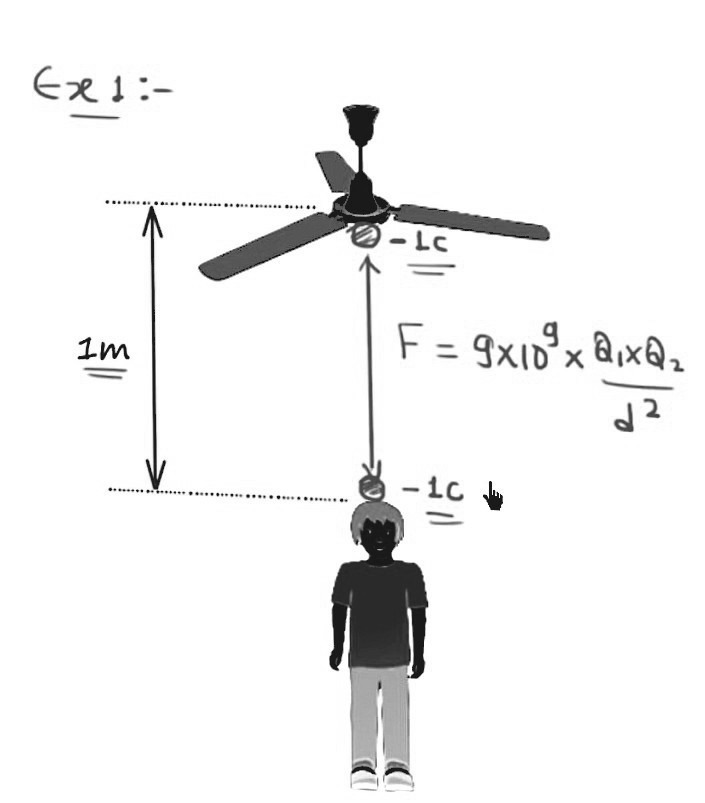
The diagram has one ceiling fan, and one boy is standing just below the ceiling fan. And the distance between the ceiling fan and the boy is equal to 1 metre. And now we will put a one-coulomb charge on the fan, and the charge is negative.
We will put a one-coulomb charge on the head of the boy. Now, this boy looks very calm, and he’s enjoying his time. But, he should be running because he will experience a very large amount of force.
He will experience the force because we know when two similar charges are placed near, they will replace each other, and therefore There will be a force of repulsion. And this force of repulsion will be equal to
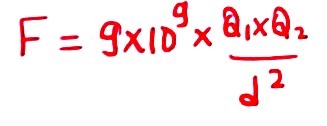
And we are writing this using Coulomb’s law and
- Q1 is equal to -1C,
- Q2 is equal to -1C.
- distance is equal to 1 meter.
So when you solve this, you will get the force. The force of repulsion is equal to 9 * 109 Newton. So this point will be a downward force equal to 9*109 Newton. And this force is equal to 9 billion newtons. And we know one Newton is approximately equal to 100 grammes. This makes 9 billion Newton approximately equal to 900 million kilogrammes.
So the boy will feel the weight equal to 900 million kilogrammes on his head. So now you can have the feeling that how large Coulomb is.
Our Another Domains Vidyapedia.in And Uietmdu.in